Question 824242: When added to 100 g of water, which of the following solutions will have the greatest boiling point elevation when compared to the boiling point of the pure solvent? (1 point)
Select one:
a. 1.75 moles of calcium fluoride (CaF2)
b. 2.0 moles of sodium chloride (NaCl)
c. 2.50 moles of potassium chloride (KCl)
d. 3.5 moles of glucose (C6H12O6)
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! In reality, I would expect none of the stated amounts to dissolve in 100g of water. Not even half of those amounts.
The expected answer may be
a. 1.75 moles of calcium fluoride (CaF2),
because, IF fully dissolved, it would provide
 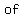 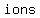 . .
That is a big IF.
My handbook says that 0.0017 parts of  will dissolve in 100 parts of hot water. will dissolve in 100 parts of hot water.
That would be 0.0017g  in 100g (100mL) of hot water. in 100g (100mL) of hot water.
(The solubility in cold water is listed as 0.0016 parts per 100 parts).
That would mean that you could not dissolve 1 mol  (78 g (78 g  ) in 100 mL water. ) in 100 mL water.
You could add 1.75 moles, but very little of it will dissolve.
Those 1.75 moles would not even dissolve in a bathtub full of water.
On the other hand, sodium chloride, potassium chloride, and glucose are very soluble in water, and you would get more dissolved.
If you could use more water, you may get the whole amount dissolved for the amounts given.
My handbook says that you can dissolve
39.8g NaCl (formula weight=58.45 g/mol) in 100 g boiling water,
56.7g KCl (formula weight=74.56 g/mol) in 100 g boiling water, and
82g glucose (molecular weight=180 g/mol) in 100 g water (temperature not specified).
From those numbers, you would need 294,329, and 769 g of water to dissolve the given amounts of NaCl, KCl, and glucose respectively.
Given a reasonable amount of water, such as 1000 g water, the NaCl, KCl, glucose would fully dissolve, and
b. 2.0 moles of sodium chloride (NaCl) will produce 4.0 moles of ions (2 ions per molecule)
c. 2.50 moles of potassium chloride (KCl) will produce 5.0 moles of ions (2 ions per molecule)
d. 3.5 moles of glucose (C6H12O6) will produce 3.5 moles of undissociated glucose molecules.
In that case, I would expect the boiling point elevation to be greatest when 2.5 moles of potassium chloride are dissolved in the same amount of water, because boiling point elevation is proportional to molal concentration of dissolved particles (molecules or ions).
TIP: The website mychemistrytutor is a good site for chemistry free help.
(I used to try to help there, until I figured that help was more desperately needed here).
|
|
|