Question 983122: A mixture of nitrogen,oxygen,and helium contains 0.25 ,0.15 and 0.4 respectively if d pressure cionsituted by oxgen is 2.5 atmosphere find partial pressure of helium?
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! Where are the units for 0.25 ,0.15 and 0.4?
If a mixture of nitrogen, oxygen, and helium contains 0.25, 0.15 and 0.4  respectively, respectively,
and the partial pressure of oxygen is 2.5 atmosphere,
then the partial pressure of helium is  {rounded to a reasonable number of significant digits). {rounded to a reasonable number of significant digits).
If the mixture of nitrogen, oxygen, and helium contains 0.25, 0.15 and 0.4  respectively, respectively,
the answer is 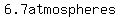 too. too.
If the mixture of nitrogen, oxygen, and helium contains 0.25, 0.15 and 0.4  respectively, respectively,
then the number of moles is
 for oxygen, and for oxygen, and
 for helium, for helium,
and the partial pressure of helium is

|
|
|