Question 950986: the rate of a chemical reaction varies, depending on the initial amount of a certain compound. In general, the reaction rate, V, can be expressed by the following formula, where k is a positive constant, and a is the initial amount of the compound. v(x)=kx(a-x) The positive constant, k, for one such reaction is 0.6, and the initial amount of the compound, a, is 18. For what value of x is the rate a maximum.
Answer by ankor@dixie-net.com(22740)   (Show Source): (Show Source):
You can put this solution on YOUR website! the rate of a chemical reaction varies, depending on the initial amount of a certain compound.
In general, the reaction rate, V, can be expressed by the following formula, where k is a positive constant, and a is the initial amount of the compound.
v(x)=kx(a-x) The positive constant, k, for one such reaction is 0.6, and the initial amount of the compound, a, is 18.
For what value of x is the rate a maximum.
v(x) = .6x(18 - x)
v(x) = 10.8x - .6x^2
The maximum will occur at the axis of symmetry, x = -b/(2a)
x = 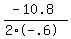
x = 9 for max volume as you can see when you graph this equation
:

|
|
|