Question 934841: If you have ever swum in a pool and your eyes began to sting and turn red, you are aware of the effects on an incorrect pH level. The pH level measures the concentration of hydronium ions and can be modeled by the function p(t) = –log10 t. The variable t represents the amount of hydronium ions, and p(t) gives the resulting pH level.
Water at 25 degrees Celsius has a pH of 7. Anything that has a pH lower than 7 is called acidic, while a pH above 7 is basic, or alkaline. Seawater has a pH just over 8, while lemonade has a pH of approximately 3.
Create a graph of the pH function. Locate on your graph where the pH value is 0 and where it is 1. You may need to zoom in on your graph.
A pool company forgets to bring their logarithmic charts, but they need to raise the amount of hydronium ions in a pool by 0.50. Using complete sentences, explain how your graph can be used to solve 10–y= 0.50. Find the approximate solution.
The pool company has developed new chemicals that transform the pH scale. Using the pH function p(t) = –log10 t as the parent function, explain which transformation would result in a y-intercept. Use complete sentences and show all translations on your graph.
Answer by josgarithmetic(39618)   (Show Source): (Show Source):
You can put this solution on YOUR website! Fix the notation.
t, the concentration of hydronium in MOLES per LITER;
p(t), intended to mean pH, negative logarithm, base ten, of the concentration t;
 . .
Use graph paper. Use a set of molarities starting from 1.0 and reaching down to 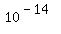 , in decreasing powers of 10, NOT in integer values of molarity. As an alternative, you could use semi-log paper, if you know how. , in decreasing powers of 10, NOT in integer values of molarity. As an alternative, you could use semi-log paper, if you know how.
|
|
|