Question 933473: A science teacher has a supply of 5% hydrochloric acid and a supply of 65% hydrochloric acid (HCl). How much of each solution should the teacher mix together to get 42 mL of 45% HCl for an experiment?
I tried to solve this but I got crazy decimals. I do not understand. I'm thinking your're going to have to turn the percents into decimals but I'm not sure.
Found 2 solutions by josgarithmetic, MathTherapy:
Answer by josgarithmetic(39618)   (Show Source): (Show Source):
Answer by MathTherapy(10552)   (Show Source): (Show Source):
You can put this solution on YOUR website!
I tried to solve this but I got crazy decimals. I do not understand. I'm thinking your're going to have to turn the percents into decimals but I'm not sure.
What type of crazy decimals did you get? Why didn't you share them?
Let amount of 5% acid to be mixed, be F
Then amount of 65% acid to be mixed = 42 F
Therefore, we get: .05F + .65(42 F) = .45(42)
.05F + 27.3 - .65F = 18.9
.05F - .65F = 18.9 27.3
- .6F = - 8.4
F, or amount of 5% acid to be mixed = 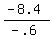 , or , or  mL mL
Amount of 65% acid to be mixed: 42 14, or  mL mL
You can do the check!!
===================
If you need a complete and detailed solution, let me know!!
Send comments, thank-yous, and inquiries to D at MathMadEzy@aol.com.
Further help is available, online or in-person, for a fee, obviously.
For FREE info and answers to questions about the ASVAB exam, the NYS 3 8 city/state wide exams,GENERAL
MATH and HOMEWORK QUESTIONS, or MATH QUESTIONS related to the Regents Integrated Algebra,
Regents Geometry, Regents Algebra 2/Trigonometry, SHSAT, COOP/HSPT/TACHS, PSAT, SAT, ACT, SSAT/ISEE,
GRE, CLEP, and the TASC/GED, you can visit: http://asvabstudyzone.freeforums.net/.
|
|
|