Question 928062: How much heat is needed to raise the temperature of a metal from 20 degrees celcius to 250 degrees celcius? The Specific heat of metal is 0.40 J/g-K.
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! You need to know how much metal you are heating.
Naturally, you need more heat to heat a kilogram of metal than to heat a gram of metal.
The temperature change, in degrees Celsius, is  . .
The temperature change, in Kelvins, is also 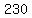 , because the Celsius scale is the same as the Kelvin scale, just shifted. , because the Celsius scale is the same as the Kelvin scale, just shifted.
To heat  grams of that metal by grams of that metal by 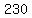 kelvins, kelvins,
you would need 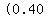 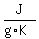 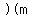    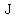 . .
So, for 10 grams of metal, the heat needed would be 920 Joules.
|
|
|