A pharmacist wants to prepare 300 ml of a 20 percent alcohol solution. How much of a 30 percent solution and a 15 percent solution can be used to form the desired mixture?
Let amount of 30% solution to be mixed be T
Then amount of 15% solution to be mixed = 300 – T
Therefore, we have: .3(T) + .15(300 – T) = .2(300)
.3T + 45 - .15T = 60
.3T - .15T = 60 – 45
.15T = 15
T, or amount of 30% solution to be mixed = 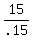 , or
, or  mL
mL
Amount of 15% solution to be mixed = 300 – 100, or  mL
mL