Question 883542: I have a bag of zinc powder i have to mix with liquid binder the ratio is 77% liquid to 23% powder. The total bag of powder mixed with liquid makes 20 litres
I need to mix batches of 2 or maybe 3 litres at a time. How do i calculate. I know i must obviously weigh the whole bag of powder first. Then add the correct weight to the correct liquid?
Found 2 solutions by ankor@dixie-net.com, KMST:
Answer by ankor@dixie-net.com(22740)   (Show Source): (Show Source):
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! The instruction would be more reasonable if it said to mix 23 parts of the powder with 77 parts of the liquid.
In that case, I would interpret "parts" as quantities measured by volume, unless people using those products are expected to weigh the materials accurately.
Mixing is a tricky business, because the final mix volume is often not the sum of the volumes mixed.
You could ask a chemist, and I am one.
However, I have more questions than answers.
MY BEST GUESS:
Maybe you can try this for your first 2-Liter batch, or maybe you could make an even smaller batch first.
Hopefully very precise mixing is not crucial to what you are doing.
IF (big IF) the liquid does not react with the powder, and/or dissolve it,
and/or retain air bubbles, you would get a slurry,
and mixing 23 parts of powder (measured by volume) with 77 parts of liquid (measured by volume) would give you
about 88 parts (by volume) of the slurry.
That is because there is always air between the particles of a powder,
and if the powder does not dissolve, or react, and air is not retained as bubbles, the final volume will be the sum of the volumes measures minus the volume of the air that was in between the particles of the powder (which would be about half of the volume of the powder).
Then, to make 2 Liters (which would be 88 parts),
you would have  , and you would use , and you would use
 of powder, along with of powder, along with
 of liquid. of liquid.
That means mixing about a half Liter of powder with 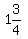 liters of liquid. liters of liquid.
To make 3 Liters be the total 88 parts of the mix,
you would mix  of powder (about (3/4 Liter), with of powder (about (3/4 Liter), with
 (about (about 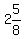 or or 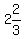 Liters) of liquid. Liters) of liquid.
THE QUESTIONS:
What is meant by a ratio of 77% liquid to 23% powder?
If a 23% and a 77% must add to 100%, they should be measure the same way,
both as volumes, or both as weights.
Do they want you to weigh the powder and the liquid?
Do they mean you get some measuring cup;
you fill it to a volume of 23 fluid ounces;
pour that into a container;
then measure 77 fluid ounces of liquid;
then add it to the container,
and mix it with the powder?
Do they want you to weigh the powder and the liquid?
The major issue is what will happen when powder and liquid mix.
Whether the powder dissolves completely, partially, or not at all, you will end up with a final volume that is neither the sum of both volumes, nor the volume of the liquid.
That happens when you mix a cup of sugar with 3 cups of water {you get about  cups of sweet solution). cups of sweet solution).
When you mix one cup of gravel with 3 cups of water in a fish tank, you get more than 3 cups of total volume, but the total volume is less than 4 cups, because part of the 1 cup of gravel was air gaps between the gravel pieces.
In the case of gravel (or any powder that does not dissolve) I may be able to find the bulk density and/or the density or specific gravity of the solid in a table in some book, or in some internet website.
For example, 1 cubic centimeter of solid zinc metal (a solid block) weighs 7.14 grams, but powder or dust is about half air. Franklimmiller.com reports the bulk densities of materials. According to their table, the weight of a cubic centimeter if "zinc dust" is 3.2 grams, and for "zinc powder" they report 3.36 grams per cubic centimeter.
The "bulk density" is the weight of a volume of powdery material measured in standard conditions. When you measure a powder, you may be packing it a litlle less tightly or more tightly than that standard way.
Otherwise, if I weighed an amount of pure zinc powder,
I would know that the volume (in cubic centimeters) that powder would take in a liquid, if it does not dissolve, is the weight divided by 7.14.
If a cubic centimeter of zinc powder weighs 3.36g, but 7.14g is the weight of 1 cubic centimeter of solid zinc, then the actual zing in that powder is
 , meaning that 53% of that zinc powder is air. , meaning that 53% of that zinc powder is air.
|
|
|