Question 790009: Stuck need help with this problem:
A chemist needs a 110 mL of 32% solution, but only has 29% & 40% solutions available. How much of each solution does he need?
Can you please walk me thru this to help me get the answer?
Found 2 solutions by josgarithmetic, MathTherapy:
Answer by josgarithmetic(39618)   (Show Source): (Show Source):
You can put this solution on YOUR website! {amount of dissolved component}/{amount of resulting solution}={concentration of dissolved component}
AND
{sum of quantities of all solutions} = {total amount of resulting solution}
x = volume of 29% solution
y = volume of 40% solution
 and and 
Answer by MathTherapy(10552)   (Show Source): (Show Source):
You can put this solution on YOUR website!
Stuck need help with this problem:
A chemist needs a 110 mL of 32% solution, but only has 29% & 40% solutions available. How much of each solution does he need?
Can you please walk me thru this to help me get the answer?
Let amount of 29% to be used be T
Then amount of 40% to be used = 110 T
Therefore, .29T + .4(110 T) = .32(T + 110 T)
.29T + 44 - .4T = .32T + 35.2 - .32T
.29T - .4T = 35.2 44
- .11T = - 8.8
T, amount of 29% solution to be used = 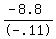 , or , or 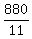 , or , or  mL mL
Amount of 40% solution to be used = 110 - 80, or  mL mL
You can do the check!!
Send comments, thank-yous, and inquiries to D at MathMadEzy@aol.com. Further help is available, online or in-person, for a fee, obviously.
For FREE info and answers to questions about the ASVAB exam, the NYS 3 8 city/state wide exams, GENERAL MATH QUESTIONS, or MATH QUESTIONS related to the Regents Integrated Algebra, Regents Geometry, Regents Algebra 2/Trigonometry, SHSAT, COOP/HSPT/TACHS, PSAT, SAT, ACT, SSAT/ISEE, GRE, CLEP, and the GED, you can visit: http://asvabstudyzone.freeforums.net/
|
|
|