Question 541107: I need to help my daughter with this question. When iron metal reacts with oxygen, the reaction can form Fe203. The balanced chemical equation I think is right is 4 Fe + 302----2Fe203. So I have 4 moles of iron + 3 moles of oxygen creates 2 moles of iron oxide. I need to find the number of moles of oxygen that are needed to form 6 mol of Fe203. Could you show all work so I can understand? I have looked through internet to try and understand and also in her pre algebra book but the use only h20 so I dont get it. I know 1 mole =6.02*10^23 so confused. Thank you if you can help.
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! You do not need to worry about the mole. It's just a ratios and proportions math problem trying to imitate a real life problem, and just confusing everybody.
Your reaction is properly balanced. I hope they gave it to you already balanced, and were not trying to torture you too much.
Mole is a name like dozen, or gross, that means a set of certain number of objects. The difference is that it is a really large number.
Instead of 12 items (as in dozen) or 144 (as in gross) a mole is 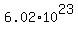 items. items.
But you do not need to care about the number, because it is the same number for everything. As a consequence, the balanced reaction tells you that 4 atoms of iron react with 3 molecules of oxygen to yield 2 formula units of Fe2O3, but it also means that 4 moles of iron react with 3 moles of oxygen to yield 2 moles of Fe2O3.
I the mychemistrytutor website, I would explain it like this:
From the coefficients in the balanced reaction, we know that the mole ratio is
3 mol oxygen/2 mol Fe2O3
(The symbol for the unit mole is just mol without the "e").
The get 6 moles Fe2O3, we need:
(6 mol Fe2O3)x(3 mol oxygen/2 mol Fe2O3) = 9 mol oxygen
The concept of mole arose from the fact that a few atoms and molecules were too small an amount to work with. Chemists had figured out the mass of all atoms relative to the mass of the smallest atom, the hydrogen atom. Amounts measured in those relative units, called atomic mass units (amu) or Daltons, were too small for general purposes. So they decided to scale up and say that if the average mass for an atom of iron is 55.845 amu, then a 55.845 grams of amount of iron will be called a mole of iron atoms. Then they carefully calculated that 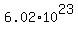 conversion factor between mole and amu, that is probably only used in some highly theoretical work. conversion factor between mole and amu, that is probably only used in some highly theoretical work.
KMST (which really stands for chemist, not my initials)
|
|
|