Question 1201552: The radioactive isotope of potassium-42, which is vital in the diagnosis of brain tumors, has a half-life of 12.36 hours.
If 500-mg of potasium-42 was taken, how many milligrams of this isotope will remain after 48 hours?
Found 2 solutions by josgarithmetic, ikleyn:
Answer by josgarithmetic(39621)   (Show Source): (Show Source):
Answer by ikleyn(52817)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The radioactive isotope of potassium-42, which is vital in the diagnosis of brain tumors,
has a half-life of 12.36 hours.
If 500-mg of potasium-42 was taken, how many milligrams of this isotope will remain after 48 hours?
~~~~~~~~~~~~~~~~~~~~~~~~~~~
Since you are given the half-life time period of 12.36 hours,
use the standard exponential decay formula with the base (1/2)
m(t) = 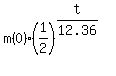 ,
where m(0) is the starting mass at t= 0 and m(t) is remaining mass in the current time moment t,
t is the time in hours.
To answer the problem's question, calculate the remaining mass at t = 48 hours using the formula
m(t) = ,
where m(0) is the starting mass at t= 0 and m(t) is remaining mass in the current time moment t,
t is the time in hours.
To answer the problem's question, calculate the remaining mass at t = 48 hours using the formula
m(t) =  = 33.8783 grams (rounded). ANSWER = 33.8783 grams (rounded). ANSWER
Solved, answered and explained.
-----------------
On radioactive decay, see the lesson
- Radioactive decay problems
in this site.
You will find many similar (and different) solved problems there.
Use this lesson as your handbook, textbook, guide, tutorials, and (free of charge) home teacher.
Learn the subject from there once and for all.
////////////////////
The major reason, why I started write this my post, is to teach you that in radio-active decay problems,
when the half-life is given, you should use the exponential decay model with the base 1/2,
which leads you to the end and to the answer by a SHORTEST way.
It is not only the SHORTEST way in such problems, but it is the EXPECTED way, too.
Finally, it is not only the shortest way and not only the expected way:
it is a GOOD STYLE way, which demonstrates
that you do understand the subject in full and PRECISELY as it is.
|
|
|