.
A certain gas sample has a volume of 40.00 L at 273 K. At constant pressure the volume increase to 50.00 L.
What will be the final temperature of the gas?
~~~~~~~~~~~~~~~~~
Use the Charles' law. It states that under constant pressure, an ideal gas' volume is proportional
to its absolute temperature. The volume of a gas at constant pressure increases linearly with
the absolute temperature of the gas. The formula he created was
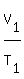 =
= 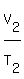 ,
where T denotes absolute temperature (in Kelvins).
In your case,
,
where T denotes absolute temperature (in Kelvins).
In your case, 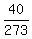 =
= 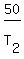 .
From the proportion,
.
From the proportion,  =
=  = 341.25 K. ANSWER
= 341.25 K. ANSWER
Solved and thoroughly explained.