Question 1195168: The efficiency of an engine is the proportion of its fuel energy that the engine can convert into motion energy. The equation E=1-x/p relates an engine’s efficiency, E, to its exhaust temperature x, in kelvins, and its operating temperature p, in kelvins. A particular automobile engine has an operating temperature of 473 kelvins and an exhaust temperature of 293 kelvins. Based on the engine’s efficiency, about how many joules of motion energy can the engine obtain from 40,000,000 joules of fuel energy?
Answer by ikleyn(52792)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
First, calculate the engine efficiency using the given formula and given data
E = 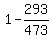 = 0.38.
After that, find joules of motion energy
joules of motion energy = 40,000,000*0.38 = 15,200,000. ANSWER = 0.38.
After that, find joules of motion energy
joules of motion energy = 40,000,000*0.38 = 15,200,000. ANSWER
Solved and explained, step by step.
|
|
|