Question 1188338: A 700-gram brine solution contains 20% salt by mass. If 300 grams of water is evaporated
from it, what percent of the new brine solution is salt?
Found 2 solutions by Theo, ikleyn:
Answer by Theo(13342)   (Show Source): (Show Source):
You can put this solution on YOUR website! the solution is 20% salt by mass.
the total solution is 700 grams.
20% * 700 = .20 * 700 = 140 grams of salt.
the rest is water.
80% * 700 = .80 * 700 = 560 grams of water.
if 300 grams of water is evaporated, the solution is left with 260 grams of water and 140 grams of salt.
the percent of salt now becomes 140 / (140 + 260) = 140 / 400 = .35 = 35% salt by mass.
Answer by ikleyn(52776)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
A 700-gram brine solution contains 20% salt by mass. If 300 grams of water is evaporated
from it, what percent of the new brine solution is salt?
~~~~~~~~~~~~~~~~~
Let me explain you, what will happen IN REALITY with the salt and with the brine solution.
From Wikipedia, the free Encyclopedia https://en.wikipedia.org/wiki/Brine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts,
brine may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater,
on the lower end of that of solutions used for brining foods) up to about 26% (a typical saturated solution,
depending on temperature). Brine forms naturally due to evaporation of ground saline water but
it is also generated in the mining of sodium chloride.[1] Brine is used for food processing and cooking
(pickling and brining), for de-icing of roads and other structures, and in a number of technological processes.
It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment
for proper disposal or further utilization (fresh water recovery).
In the course of water evaporating, the process will go smoothly until the salt concentration will reach
the value of 26%, approximately.
At this point, the brine solution becomes SATURATED and its concentration does not change any more.
The exceed mass of the salt will go as a sediment ( ! not a mixture ! ) at the bottom of the reservoir.
The concentration of the salt in the brine will remain 26%.
See this Wikipedia article https://en.wikipedia.org/wiki/Brine
It is the process which students learn in the middle school in their standard Science class.
/////////////
Nevertheless, although the process seems to be complicated and is non-linear,
it can be computed, in full, to the end. This computation is given below.
700-grams brine solution contains 20% salt by mass.
So, there are 0.2*700 = 140 grams of salt in the solution, at the beginning,
and 700-140 = 560 grams of water, correspondingly.
After 300 grams of water evaporated, 560-300 = 260 grams of water remained.
This amount of liquid contains 26% of salt, by mass
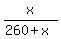 = 0.26,
which gives x = 91.35 grams of salt dissolved.
The rest of salt, 140 grams - 91.35 grams = 48.65 grams, went from the original brine solution to the sediment.
ANSWER. After 300 grams of water evaporated, the remained brine contains 260 grams of water and 91.35 grams of salt.
This brine is saturated at 26% concentration. The amount of salt of 48.65 grams went from the original brine solution to the sediment. = 0.26,
which gives x = 91.35 grams of salt dissolved.
The rest of salt, 140 grams - 91.35 grams = 48.65 grams, went from the original brine solution to the sediment.
ANSWER. After 300 grams of water evaporated, the remained brine contains 260 grams of water and 91.35 grams of salt.
This brine is saturated at 26% concentration. The amount of salt of 48.65 grams went from the original brine solution to the sediment.
Solved, answered and explained.
|
|
|