Question 1182725: Equal amounts of heat are added to equal masses of aluminum and iron at the same Initial temperature. Which metal will have the higher final temperature and how much greater will that temperature change be than the temperature change of the other metal
Found 2 solutions by Boreal, ikleyn:
Answer by Boreal(15235)   (Show Source): (Show Source):
You can put this solution on YOUR website! The specific heat of aluminum is twice as much as that for iron.
That means that aluminum will heat up twice as fast as iron for the same amount of heat. Iron is more dense and the heat will go also to the heavier atoms as well as to their motion. The atoms are lighter in aluminum.
Answer by ikleyn(52922)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
Equal amounts of heat are added to equal masses of aluminum and iron at the same Initial temperature.
Which metal will have the higher final temperature and how much greater will that temperature change be
than the temperature change of the other metal
~~~~~~~~~~~~~~~~
To get the correct answer, read the solution by @Boreal and then turn it inside out.
EXPLANATION
The specific heat of aluminum is 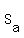 = 0.902 J/(g°C).
The specific heat of iron is = 0.902 J/(g°C).
The specific heat of iron is 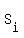 = 0.450 J/(g*°C).
(see this web-site https://cpanhd.sitehost.iu.edu/C101webnotes/matter-and-energy/specificheat.html )
It means that you need 0.902 joules of heat to increase the temperature of one gram of aluminum by 1 °C,
and you need 0.450 joules of heat to increase the temperature of one gram of iron by 1 °C.
The basic formula is Q = S*m*dT,
where Q is the amount of heat (energy); S is the specific heat per gram of mass, m is the mass in grams;
dT is increase (the change) of the temperature.
In this problem, we are given the same amount of heat energy (let say, 1 joule);
the same mass of two materials (let say, 1 gram);
so we have
dT = = 0.450 J/(g*°C).
(see this web-site https://cpanhd.sitehost.iu.edu/C101webnotes/matter-and-energy/specificheat.html )
It means that you need 0.902 joules of heat to increase the temperature of one gram of aluminum by 1 °C,
and you need 0.450 joules of heat to increase the temperature of one gram of iron by 1 °C.
The basic formula is Q = S*m*dT,
where Q is the amount of heat (energy); S is the specific heat per gram of mass, m is the mass in grams;
dT is increase (the change) of the temperature.
In this problem, we are given the same amount of heat energy (let say, 1 joule);
the same mass of two materials (let say, 1 gram);
so we have
dT = 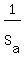 = = 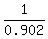 = 1.109 °C for aluminum,
and
dT = = 1.109 °C for aluminum,
and
dT =  = = 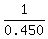 = 2.222 °C for iron.
So, the ANSWER is: of the two materials, aluminum and iron, the iron will have more high temperature
and the temperature change of IRON will be MORE THAN TWICE the temperature change of aluminum. = 2.222 °C for iron.
So, the ANSWER is: of the two materials, aluminum and iron, the iron will have more high temperature
and the temperature change of IRON will be MORE THAN TWICE the temperature change of aluminum.
One more time : the answer and the explanations in the post by @Boreal BOTH are incorrect.
The real and actual response of materials is OPPOSED to what @Boreal writes in his post.
|
|
|