.
 = 0.1
3.6 = 0.1*(20+w)
3.6 = 2 + 0.1w
3.6 - 2 = 0.1w
1.6 = 0.1w
w =
= 0.1
3.6 = 0.1*(20+w)
3.6 = 2 + 0.1w
3.6 - 2 = 0.1w
1.6 = 0.1w
w = 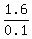 = 16.
ANSWER. 16 mL of water should be added.
CHECK.
= 16.
ANSWER. 16 mL of water should be added.
CHECK. 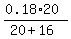 =
= 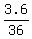 = 0.1 = 10% concentration. ! Correct !
= 0.1 = 10% concentration. ! Correct !
It can be solved mentally, too.
20 mL of the 18% solution contain 0.18*20 = 3.6 mL pure alcohol.
To have 10% solution, the total volume must be 36 mL;
hence, 16 = 36-20 milliliters of water must be added.
The second solutions provides THE SAME answer.