The pressure of the gas is directly proportional to the temperature and inversely proportional to
its volume.
A. Write the variation equation.
For all proportion problems, start with this:
Varying "directly" or product of "jointlys" or 1 if none
quantity = k × ----------------------------------------------------------
inversely variable or product of "inverselys" or 1 if none
In this problem the varying quantity is P for pressure. The "directly" is T for
temperature. We have one "inversely" variable, T for Volume. We have no
"jointly" variable. So we have P on the left, T on the top of the fraction and
V on the bottom:
 -----------------------
-----------------------
B. What will happen to the pressure if the volume is reduced to half and the
temperature is doubled?
Let's substitute 2V for V in  , and
, and 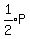 for P and simplify:
for P and simplify:
 We simplify the complicated term
We simplify the complicated term 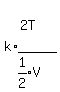

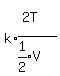

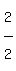

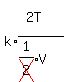


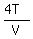 Since
Since  is 4 times
is 4 times  , the answer to B is:
"The pressure will quadruple."
Edwin
, the answer to B is:
"The pressure will quadruple."
Edwin