.
The decay equation in this case is
M = 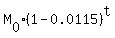 =
=  ,
where
,
where 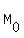 is the original mass, M is the current mass, t is the time in days.
They want you find "t" from the condition M =
is the original mass, M is the current mass, t is the time in days.
They want you find "t" from the condition M = 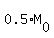 .
It gives
0.5 =
.
It gives
0.5 =  .
Take the logarithm base 10 from both sides
log(0.5) = t*log(0.9885).
Hence, t =
.
Take the logarithm base 10 from both sides
log(0.5) = t*log(0.9885).
Hence, t = 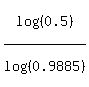 = 59.93 = 60 days (rounded as required). ANSWER
= 59.93 = 60 days (rounded as required). ANSWER
Solved.