.
In this problem, concentration is measured in grams per milliliter, g/mL.
// Not in grams per grams (!) It is important (!) And it is important to understand (!)
You can solve it using simple logic.
100 ml of the 10% salt solution contain 0.1*100 = 10 grams of dissolved salt.
In order 10 grams of dissolved salt make 8% solution, the volume of the solution must be 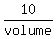 = 0.08, i.e. volume =
= 0.08, i.e. volume = 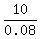 = 125 mL.
So you need add 125-100 = 25 mL of the sterile water. ANSWER
= 125 mL.
So you need add 125-100 = 25 mL of the sterile water. ANSWER
Or you can solve it using Algebra.
Let W be the volume of the sterile water to add.
Then the total volume is (100+W) milliliters, and your equation is
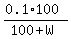 = 0.08,
saying that the final concentration is 8%.
Solve it. First step multiply both sides by (100+W).
0.1*100 = 0.08(100+W)
10 = 8 + 0.08W
10 - 8 = 0.08W
2 = 0.08*W
W =
= 0.08,
saying that the final concentration is 8%.
Solve it. First step multiply both sides by (100+W).
0.1*100 = 0.08(100+W)
10 = 8 + 0.08W
10 - 8 = 0.08W
2 = 0.08*W
W = 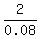 = 25.
You get the same ANSWER : 25 mL of the sterile water should be added.
= 25.
You get the same ANSWER : 25 mL of the sterile water should be added.
Solved (in two ways).
-----------------------
It is a typical and standard mixture problem.
There is a bunch of introductory lessons in this site, covering various types of mixture problems
- Mixture problems
- More Mixture problems
- Solving typical word problems on mixtures for solutions
- Word problems on mixtures for antifreeze solutions
- Word problems on mixtures for alloys
- Typical word problems on mixtures from the archive
Read them and become an expert in solution the mixture word problems.
Also, you have this free of charge online textbook in ALGEBRA-I in this site
- ALGEBRA-I - YOUR ONLINE TEXTBOOK.
The referred lessons are the part of this online textbook under the topic "Mixture problems".
Save the link to this online textbook together with its description
Free of charge online textbook in ALGEBRA-I
https://www.algebra.com/algebra/homework/quadratic/lessons/ALGEBRA-I-YOUR-ONLINE-TEXTBOOK.lesson
to your archive and use it when it is needed.