.
(A) To answer (A), divide the mass of 8.36 grams of HCl by 20 mL
 = 0.418 g of HCl per milliliter of acid solution.
(B) Question (B) is formulated unclear in the post. It does not says explicitly, to which mass to relate 8.36 g of HCl.
But from the context, there is only ONE such a mass: it is the total mass of the solution (20 mL * 1.18 g/mL).
So, from the context the reader should guess it.
Thus, to answer (B), divide the given mass of 8.36 grams of HCl by the total mass of the acid solution, which is (20 mL * 1.18 g/mL).
Then multiply by 100% to get percentage :
the percent by weight (mass) of HCl in the concentrated hydrochloric acid =
= 0.418 g of HCl per milliliter of acid solution.
(B) Question (B) is formulated unclear in the post. It does not says explicitly, to which mass to relate 8.36 g of HCl.
But from the context, there is only ONE such a mass: it is the total mass of the solution (20 mL * 1.18 g/mL).
So, from the context the reader should guess it.
Thus, to answer (B), divide the given mass of 8.36 grams of HCl by the total mass of the acid solution, which is (20 mL * 1.18 g/mL).
Then multiply by 100% to get percentage :
the percent by weight (mass) of HCl in the concentrated hydrochloric acid = 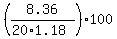 = 35.42% (approximately).
= 35.42% (approximately).
Solved, answered and explained.