Question 1139500: A pot of boiling soup with an internal temperature of 100° Fahrenheit was taken off the stove to cool in a 67°F room. After fifteen minutes, the internal temperature of the soup was 95°F.
To the nearest minute, how long will it take the soup to cool to 85°F?
Found 4 solutions by Theo, MathTherapy, Alan3354, ikleyn:
Answer by Theo(13342)   (Show Source): (Show Source):
Answer by MathTherapy(10555)   (Show Source): (Show Source):
You can put this solution on YOUR website!
A pot of boiling soup with an internal temperature of 100° Fahrenheit was taken off the stove to cool in a 67°F room. After fifteen minutes, the internal temperature of the soup was 95°F.
To the nearest minute, how long will it take the soup to cool to 85°F?
Using Newton's Law of Cooling - any of the following 3 formulas:  , you , you
should find that it'll take the soup: 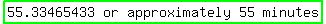 to cool to a temperature of 85oF. to cool to a temperature of 85oF.
Answer by Alan3354(69443)   (Show Source): (Show Source):
You can put this solution on YOUR website! It is possible for water (or soup) to boil at 100 degs F at low pressure, but no one lives at an altitude of 51,160 feet.
-------
IDK what the pressure is inside the ISS, but I don't think they make soup anyway.
=========
It's true, Theo used an inapplicable method.
We should have him killed.
Or maybe just deported, but we don't know where he lives, or to where we could deport him.
Maybe to somewhere at an altitude of 51,000 feet?
=============
PS Years ago, when I was studying calculus, I figure out the cooling thing.
I thought I had discovered something new, but then found that I was 300 or 400 years too late.
I coulda been first, but I wasn't yet born.
Answer by ikleyn(52812)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The solution by @Theo is wrong, since he uses a linear function for the temperature decrease in time.
while this function must be shifted exponent, according to the Newton's cooling law.
I even have special lesson with a template solution for this kind of problems,
but for the person, who is not able to distinct Fahrenheit and Celsius degrees I don't want even show the link to this lesson.
There is no any sense to explain something relevant to such a person.
/\/\/\/\/\/\/\/
Special addition for Alan : the air pressure inside ISS is practically atmospheric, 101.3 kPa.
See this Wikipedia article https://en.wikipedia.org/wiki/ISS_ECLSS
|
|
|