.
In such problems, using exponents like 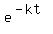 is the way to create unnecessary difficulties for yourself.
Simply use the fact that the 48 hours are 16 times half-life.
It will give you
is the way to create unnecessary difficulties for yourself.
Simply use the fact that the 48 hours are 16 times half-life.
It will give you 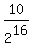 remaining mass (in kilograms) of the original material.
remaining mass (in kilograms) of the original material.
Also, to better understand the Physics (which most school students simply do not know),
keep in your mind, that actually the total mass of a sample (the bulk mass) changes INSIGNIFICANTLY at the radioactive decay.
Simply one chemical element is transforming to another chemical element / (or to its isotope).
Again, the total mass of the original sample material changes insignificantly.
Otherwise, it would be a nuclear explosion.