Question 1118213: To prepare 20 mL of a 5 mg/mL solution from a stock solution of 100 mg/mL, how much stock solution and diluent will you need?
Found 4 solutions by Boreal, greenestamps, josgarithmetic, ikleyn:
Answer by Boreal(15235)   (Show Source): (Show Source):
You can put this solution on YOUR website! 100 mg/ml*x ml=5 mg/ml*20ml. The right side is 100 mg
Therefore, the left side has to be 100 mg, and that is with 1 ml of the 100 mg/ml, which gives 100 mg.
1 ml of stock solution
19 ml of diluent, assuming the two don't interact.
Answer by greenestamps(13198)   (Show Source): (Show Source):
You can put this solution on YOUR website!
The strength of the desired solution is 1/20 of the strength of the stock solution (5 mg/mL compared to 100 mg/mL). That means 1/20 of the mixture must be the stock solution.
1/20 of 20 mL is 1 mL.
You need 1 mL of the stock solution and 1 mL of the diluent.
--------------------------
Sorry. That last line was a typo. The work I show leading up to my statement of the answer clearly shows that the "1mL" of diluent was supposed to be 19mL.
Answer by josgarithmetic(39615)   (Show Source): (Show Source):
Answer by ikleyn(52767)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
1. Algebra solution
Let S be the volume of the 100 mg/L stock solution required (in milliliters), and
let D be the volume of the diluent required.
Then
S + D = 20, (1) (counting the total liquid volume)
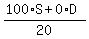 = 5. (2) (counting the concentration of the resulting mixture in milligrams per milliliters)
Simplify (2) to
100*S = 5*20 = 100,
which implies S = = 5. (2) (counting the concentration of the resulting mixture in milligrams per milliliters)
Simplify (2) to
100*S = 5*20 = 100,
which implies S = 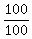 = 1.
Then from equation (1), D = 20 - S = 20 - 1 = 19.
Answer. 1 milliliter of the 100 mg/mL stock solution and 19 mL of the diluent is required. = 1.
Then from equation (1), D = 20 - S = 20 - 1 = 19.
Answer. 1 milliliter of the 100 mg/mL stock solution and 19 mL of the diluent is required.
2. Common sense solution.
To dilute the 100 mg/ML stock solution to the concentration of 5 mg/mL, you should have one volume of the stock solution
per 20 volumes of the total liquid.
In other words, you need to add 19 volumes of the diluent to each one volume of the original stock solution,
and then you will have totally 20 volumes of the mixture of the required concentration.
In our case 20 volumes are exactly 20 milliliters, which means that you need 1 milliliter of the stock solution and 19 milliliters of the diluent.
The answer by @greenestamps is a mistake.
The answers of the two other tutors are correct.
|
|
|