|
Question 1108137: What is the % w/v of 8g of dextrose in 50ml?
Found 3 solutions by Collinwilfixyouuprealnice, KMST, ikleyn:
Answer by Collinwilfixyouuprealnice(44)   (Show Source): (Show Source):
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! If you work in a life sciences lab, you find concentrations listed as %w/v sometimes.
For %w/v, we use grams an milliliters as if they were the same unit.
If the total volume of the solution is 50mL,
then the % w/v is  . .
That is where a math problem ends.
That solution will NOT weigh 58 g, so its %w/w would NOT be
 which would be which would be  . .
That solution will be more dense than water, but not by much.
Its density at common room temperatures will be 1.06 g/mL. (I look that up when I need to).
So, your 16% w/v solution would weigh  , ,
with a w/w ratio of  . .
That approximate ratio is  , and that is the %w/w. , and that is the %w/w.
A DIFFERENT PROBLEM:
However, if you mix 8g dextrose and 50mL of water, for a final weight of about  , ,
that is a different math problem.
If you are a pharmaceutical formulator or a chemical engineer that is your problem.
The final volume will be more than 50mL, but not quite 58mL.
The concentration as %w/w would be  , ,
which is 13.8%w/w.
A table of densities, or "specific gravities" would tell you that its density would be about 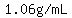 too. too.
That means its volume would be
 . .
That would make its (solute weight)/(total volume) ratio
about 
and its %w/v concentration  . .
Answer by ikleyn(52867)   (Show Source): (Show Source):
|
|
|
| |