.
I just solved and answered this problem at the link
https://www.algebra.com/algebra/homework/word/misc/Miscellaneous_Word_Problems.faq.question.1102280.html
https://www.algebra.com/algebra/homework/word/misc/Miscellaneous_Word_Problems.faq.question.1102280.html
For your convenience, I copy and paste this solution here again:
-------------------------------------------
Below is your input with my editing:
Specific gravity of gold:  19.259.
Specific gravity of quartz:
19.259.
Specific gravity of quartz:  2.652.
Determine ounces of gold in an mass of 210 oz. of both, the specific gravity is
2.652.
Determine ounces of gold in an mass of 210 oz. of both, the specific gravity is  4.562.
Specific gravity is the weight of a volume of a substance divided by the weight of the same
4.562.
Specific gravity is the weight of a volume of a substance divided by the weight of the same  volume of water.
Water weighs 62.5 lbs. per cu. ft. <<<---=== In my solution I do not need this info.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
volume of water.
Water weighs 62.5 lbs. per cu. ft. <<<---=== In my solution I do not need this info.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
First step is to find the fraction "x" of the gold in 210 oz. of mixture of the specific gravity 4.562.
For it, use the density equation
x*19.259 + (1-x)*2.652 = 4.562.
19.259x + 2.652 - 2.652x = 4.562 ====> 16.607x = 4.562 - 2.652 = 1.91 ====> x = 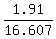 = 0.115.
So, the mass fraction of the gold in the mixture is 0.115.
Next step determine the mass of the gold in the mass of 210 oz of the mixture:
gold mass = 0.115*210 = 24.15 oz.
= 0.115.
So, the mass fraction of the gold in the mixture is 0.115.
Next step determine the mass of the gold in the mass of 210 oz of the mixture:
gold mass = 0.115*210 = 24.15 oz.
Answer. The mass of the gold is 24.15 oz.
Solved.