Question 1101751: A chemist was preparing a solution that should have included 35 milligrams of a chemical. If she actually used 36.4 milligrams, what was her percentage error (to the nearest 0.01%)
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! In absolute terms, the error was  , ,
but an error of 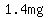 does not represent a big fraction of the does not represent a big fraction of the 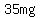 target amount. target amount.
As a fraction, it is 
A chemist would not say it should have included "35" milligrams.
If you ask for 35 mg, you have to accept 34.50mg and 35.49mg as being "35mg".
If the chemist have to be more precise than that,
you ask for 35.0mg, or 35.00 mg, with more significant digits.
You probably would say something about the amount being within 1% of 35.0 mg,
or within 0.5mg of 35.0mg, or within 0.01% of 35.000 mg,.
|
|
|