Question 1089589: The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely as the volume of the gas. If the pressure is 1170 kpa (kiloPascals) when the number of moles is 6, the temperature is 260 kelvin , and the volume is 720 cc, find the pressure when the number of moles is 3, the temperature is 280 k, and the volume is 180 cc .
Answer by ikleyn(52776)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely
as the volume of the gas. If the pressure is 1170 kpa (kiloPascals) when the number of moles is 6,
the temperature is 260 kelvin , and the volume is 720 cc, find the pressure when the number of moles is 3,
the temperature is 280 k, and the volume is 180 cc .
~~~~~~~~~~~~~~~~~~~
The condition says that
P = 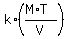 , (1)
where P is the pressure, M is the amount of the gas (measured in moles), T is the temperature, V is the volume of the gas,
and k is the proportionality coefficient.
Also, the condition says that
1170 = , (1)
where P is the pressure, M is the amount of the gas (measured in moles), T is the temperature, V is the volume of the gas,
and k is the proportionality coefficient.
Also, the condition says that
1170 = 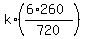 ,
which implies k = ,
which implies k = 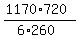 = 540.
Now, to answer the problem question, simply substitute the given data into the formula (1) and calculate the pressure:
P = = 540.
Now, to answer the problem question, simply substitute the given data into the formula (1) and calculate the pressure:
P = 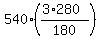 = 2520 kpa (kiloPascals). = 2520 kpa (kiloPascals).
Solved.
|
|
|