Question 1061097: if a chemist needs a 40% acid solution, how much 50% solution needs to be mixed with 8 liters of a 25% solution to obtain the desired concentration?
Found 2 solutions by josgarithmetic, KMST:
Answer by josgarithmetic(39617)   (Show Source): (Show Source):
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website!  = volume of 50% solution needed (in Liters) = volume of 50% solution needed (in Liters)
 = volume of 25% solution used (in Liters) = volume of 25% solution used (in Liters)
 = volume of the final 40% solution mixture (in Liters) = volume of the final 40% solution mixture (in Liters)
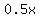 = amount of acid in = amount of acid in  Liters of 50% solution Liters of 50% solution
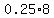 = amount of acid in = amount of acid in  Liters of 25% solution Liters of 25% solution
We need to make it so that
{{A=0.40V}}}= amount of acid in  Liters of 40% solution Liters of 40% solution
We know that
 = amount of acid in the final mixture = amount of acid in the final mixture
We assume that there was no substantial volume change on mixing, so
 = final volume (assumed, in Liters) = final volume (assumed, in Liters)
We can solve (the system of the last 3 equations) for 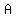 , ,  , and , and  , ,
or we can combine the 3 equations into one as
 and solve for and solve for  . .
Solving:





 . .
We need to mix  Liters of 50% solution with the 8 Liters of 25% solution to obtain the desired concentration. Liters of 50% solution with the 8 Liters of 25% solution to obtain the desired concentration.
Bonus answer:
We expect to end up with a final volume (in liters) of
 . .
NOTEs:
When you mix to solutions the final volume is not exactly the sum of the volumes mixed, but in many cases it will be close enough for your purposes
"Acid" does not identify any chemical, or mixture of compounds.
In chemistry, it identify a very broad class of chemical compounds. Exanples: ascorbic acid (also known as vitamin C), acetylsalicylic (also known as aspirin), acetic acid (the chemical compound present, along with a lot of water, in vinegar), hydrochloric acid (a solution of the gas hydrogen chloride in water, at a concentration of about 36 grams gas per 100 grams of solution), sulfuric acid (the chemical with formula 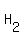 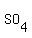 , or a solution usually containing 98 grams of that chemical per 100 grams of solution) , or a solution usually containing 98 grams of that chemical per 100 grams of solution)
In the language on non-chemists, it could mean a chemical that can cause burns and dissolve certain things, or LSD, or who knows what.
|
|
|