Question 1044097: The pressure of an ideal gas was held constant. The initial temperature and volume were 600 K and 500 ml respectively. What would the final temperature be if the volume were increased to 1500 ml?
Found 2 solutions by jorel555, ikleyn:
Answer by jorel555(1290)   (Show Source): (Show Source):
Answer by ikleyn(52786)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The pressure of an ideal gas was held constant. The initial temperature and volume were 600 K and 500 ml respectively.
What would the final temperature be if the volume were increased to 1500 ml?
~~~~~~~~~~~~~~~~~~~~~~~~~~
Apply the "Ideal gas Law".
See this Wikipedia article
The ideal gas law is the equation of state of a hypothetical ideal gas.
It is a good approximation of the behavior of many gases under many conditions, although it has several limitations.
It was first stated by Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles' law and Avogadro's Law.
The ideal gas law is often written as:
 = = 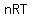 where:
P is the pressure of the gas,
V is the volume of the gas,
n is the amount of substance of gas (in moles),
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
T is the absolute temperature of the gas.
For two states of your gas
where:
P is the pressure of the gas,
V is the volume of the gas,
n is the amount of substance of gas (in moles),
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
T is the absolute temperature of the gas.
For two states of your gas
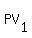 = = 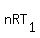 and and
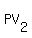 = = 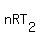 .
Now divide left sides and right sides. You will get .
Now divide left sides and right sides. You will get
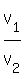 = = 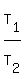 .
Hence, .
Hence,  = = 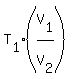 = = 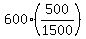 = 200 K. = 200 K.
|
|
|