Question 1033113: The container held the compound (As) sub four O sub 6
and What was the percentage by weight of the Arsenic in this compound (As, 75;O,16)
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! The formula of the compound is 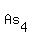 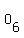 , meaning that a molecule or formula unit comprises , meaning that a molecule or formula unit comprises  atoms of atoms of 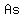 , and , and  atoms of atoms of  . .
The mass of the  atoms of atoms of 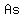 , ,
each with a mass of  atomic mass units, adds up to atomic mass units, adds up to
 . .
The mass of the  atoms of atoms of  , ,
each with a mass of  atomic mass units, adds up to atomic mass units, adds up to
 . .
The total mass of the molecule is  atomic mass units, atomic mass units,
and out of that total, the mass of the arsenic is
 atomic mass units. atomic mass units.
As a fraction, that is
 (rounding to whole numbers, or 2 significant figures, as a chemistry teacher might say). (rounding to whole numbers, or 2 significant figures, as a chemistry teacher might say).
|
|
|