Question 1007142: Q15. The half-life of a radioactive element is 130 days, but your sample will not be useful to you after 80% of the radioactive nuclei originally present have disintegrated. About how many days can you use the sample?
a. 302
b. 287
c. 312
d. 297
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! WITHOUT MEMORIZED FORMULAS:
 days is days is  half-lives, half-lives,
After that time, the amount of radioactive nuclei would have been halved  times. times.
So, if you started with an amount  , ,
after that time the amount you would have (halved  times) is times) is
 . .
You could write that as
 <--> <--> <--> <--> . .
That is what you get just by thinking it through.
It is not a formula that needs to be memorized
(unless you prefer to let other people do the thinking).
So, you could set


and substituting into  you would have you would have


If you take logarithms on both sides of the equal sign, you get an equally valid equation.
You could use logarithms on any base,
but calculators give you logarithms on bases  and and 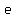 . .
So, using base 



 (rounded to nearest whole number) (rounded to nearest whole number)
THE FORMULAS:
The irrational number 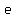 is a popular base for calculus reasons. is a popular base for calculus reasons.
So, from  , using logarithms on base , using logarithms on base 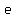 you get you get


From there, you get
 <--> <--> ,or using the approximate value ,or using the approximate value  , ,
 <--> <-->
Unfortunately, when asked to "show your work",
your teacher may expect to see one of those formulas written out,
just to prove that you are good at memorizing stuff that does not rhyme.
|
|
|