Question 1001501: If the density of a 500.0g H2SO4 solution is 1.84 g/cm^3 what are its molarity, mole fraction, and molality?
Don't know if you can help me. This is the only site I could find. It's chemistry. Do you know of a chemistry site like this one?
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! The website yeahchemistry.com will help you with chemistry questions.
If the solution was 100% sulfuric acid, you would have 500g sulfuric acid and 0g water.
When you buy a bottle of sulfuric acid it would have a label with analysis data,
and it could read "assay = 98.6%", or something like that.
However, you may assume, it is 100%, because 1.84g/mL is the densityof 100% sulfuric acid at 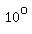 . .
(Warmer acid, and/or less concentrated acid has a lower density).
Then,  would give you the number of moles of sulfuric acid. would give you the number of moles of sulfuric acid.
You can use the density to find the volume of those 500g solution,
 . .
Then,  mol/L is the molarity, often written as mol/L is the molarity, often written as 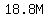 , and read as "18.8 molar". , and read as "18.8 molar".
Since  , ,  mol/kg is the molality, mol/kg is the molality,
which your instructor may express as 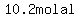 or or 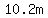 . .
The mole fraction would be
moles acid/{moles acid + moles water) ,
and since we are assuming 0% water,
that molar fraction would be 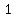 . .
|
|
|