You can
put this solution on YOUR website! .
If you add 30mL of 1:400 of Solution A to 270mL of water, what is the resulting concentration?
Select one:
Оа. 0.3%
Ob. 0.025%
Oc. 0.35%
Od. 0.15%
~~~~~~~~~~~~~~~~~~
30 mL of 1:400 of solution A contain 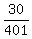 mL = 0.0748 (rounded) mL of the pure substance A.
When we add 270 mL of water, the total volume becomes 30 + 270 = 300 mL,
with the same amount 0.0748 mL of the pure substance A.
Hence, the final concentration is
mL = 0.0748 (rounded) mL of the pure substance A.
When we add 270 mL of water, the total volume becomes 30 + 270 = 300 mL,
with the same amount 0.0748 mL of the pure substance A.
Hence, the final concentration is 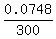 = 0.000249333 = 0.00025 (rounded) = 0.025%.
= 0.000249333 = 0.00025 (rounded) = 0.025%.
Solved and fully explained.