Question 982448: how much water is to be evaporated from 20 liters of 20 percent sugar solution to get 30 percent sugar solution.
Found 2 solutions by stanbon, KMST:
Answer by stanbon(75887)   (Show Source): (Show Source):
You can put this solution on YOUR website! how much water is to be evaporated from 20 liters of 20 percent sugar solution to get 30 percent sugar solution.
--------
Equation:
water - water = water
0.80*20 - 1*x = 0.70(20-x)
-----
Multiply thru by 100 to get:
80*20 - 100x = 70*20 - 70x
30x = 10*20
x = (1/3)20 = 6 2/3 Liters (amt. of water to evaporate)
-------------
Cheers,
Stan H.
------------
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! This is the kind of problem that makes a chemist cry (or at least hate the fact that math teacher have little knowledge of or respect for science).
THE WRONG SOLUTION THAT A MATH TEACHER EXPECTS:
 = volume of water evaporated, in L. = volume of water evaporated, in L.
The amount of sucrose in each solution is calculated by multiplying concentration times volume;
concentration used is the percentage expressed as a decimal (0.2 for 20% and 0.3 for 30%),
and the teacher does not care what units the amount of sucrose is measured in.
So, the equation is

Solving:
 --> --> --> --> --> --> --> --> --> -->
THE SCIENTIST"S (OR CHEMICAL ENGINEER'S)POINT OF VIEW:
When solution concentrations are given as percentage,
especially if it is some solid substance dissolved in water,
you would want to specify if you meant
20 grams in a total mass of 100 grams of solution, which you write as 20%(w/w),
or 20 grams in a total volume of 100 milliliters of solution, which you write as 20%(w/v),
where the "w" and the "v" refer to weight and volume respectively.
We read {w/w) as "weight in weight" and (w/v) as "weight in volume".
If someone tells me a sucrose solution's concentration is 20%,
I would assume it is 20%(w/v), with 20 g sucrose per 100 mL (100 milliliters) of solution,
and I would go to a table to find that it has a density of 1.0810 g/mL.
That means that the 100 mL of solution really have a mass of 108.1 grams,
which would make the concentration
 = 18.5%(w/w). = 18.5%(w/w).
A 30%(w/v) sucrose solution, with 30 g sucrose per 100 mL
has a density of 1.1270g/mL,
meaning that the weight ratio of sucrose to solution is
 = 26.6%(w/w). = 26.6%(w/w).
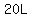 of 20%(w/v) sucrose solution have a mass of of 20%(w/v) sucrose solution have a mass of
 , ,
and would contain  sucrose. sucrose.
If  liters of 30%(w/v) sucrose solution were to contain liters of 30%(w/v) sucrose solution were to contain 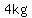 sucrose, we would have sucrose, we would have
 ---> ---> ---> ---> ---> ---> (rounded), (rounded),
and 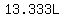 of 30%(w/v) sucrose solution have a mass of of 30%(w/v) sucrose solution have a mass of
 (rounded). (rounded).
To get to a mass of 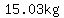 from a mass of from a mass of 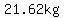 , ,
we would need to lose
 , ,
and since the lost mass is water,
with an approximate density of 1.000kg/L = 1.000g/mL,
the amount of water that needs to be evaporated is 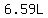
|
|
|