Question 826833: how much heat is required to convert 50g of ice at -10degree celcius to steam at 200degree celcius?
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! How much heat is required to convert 50g of ice at 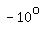 Celsius to steam at Celsius to steam at  Celsius? Celsius?
This is a problem that a science teacher (chemistry or physics) would give you.
You need to assume that the process occurs at standard atmospheric pressure (1 atm). (The pressure at start and end is really what matters, and we should assume that is is 1 atmosphere).
You need to calculate 5 different amounts of heat for 5 different stages of the process:
1) the heating of ice from r to   , ,
2) the melting at   , ,
3) the heating of the resulting liquid water from   to to   , ,
4) the vaporization of the liquid water at   , and , and
5) the heating of the resulting water vapor from   to to   . .
You need data from your textbook, or from a handbook of physics (or chemistry, or engineering).
I tried to find data, and calculate accurately, but you may have more accurate data, or be less prone to calculation mistakes, or your data may be in different units.
Water, as a liquid between   and and   , ,
has a "specific heat" of about  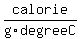 . .
As ice, a bit below   , or , or
as vapor at  to to   , ,
it has a "specific heat" of about 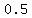 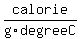 . .
That is the heat it takes to increase the temperature of 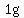 water by water by   (according to my handbook). (according to my handbook).
(Of course, the number is a function of temperature, but it does no vary by too much).
The heats, or "latent heats", of melting (for ice) and vaporization (for liquid water) are about 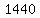 and and 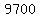 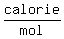 respectively (according to my handbook). respectively (according to my handbook).
So the amounts of heat required are
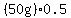 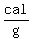 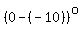  = =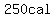 to heat the ice to to heat the ice to   , ,
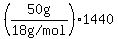  = about = about  to melt the ice at to melt the ice at   , ,
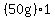 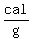 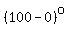  = = to heat the liquid water from to heat the liquid water from   to to   , ,
  to vaporize the water at to vaporize the water at   , and , and
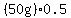 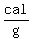 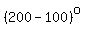  = =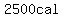 to heat the water vapor from to heat the water vapor from   to to   . .
According to my calculation, the total energy in calories is
 . .
NOTE:
Your data may slightly different, or it may be in different units (Joules rather than calories, or Joule/g rather than calorie/mol, for example).
|
|
|