Question 652559: A 20% solution of fertilizer is to be mixed with a 80% solution of fertilizer in order to get 120 gallons of a 60% solution. How many gallons of the 20% solution and 80% solution should be mixed?
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! Let the number of gallons of 20% solution of fertilizer to be used be  . .
Let the number of gallons of 80% solution of fertilizer to be used be  . .
As you mix the solutions, the volumes add up,
so the final volume (in gallons) is  . .
That is your first equation.
 gallons of a 20% solution contain gallons of a 20% solution contain 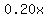 gallons of fertilizer. gallons of fertilizer.
 gallons of a 80% solution contain gallons of a 80% solution contain 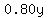 gallons of fertilizer. gallons of fertilizer.
When you mix them, the final mix will contain 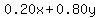 gallons of fertilizer, gallons of fertilizer,
but that must be the amount of fertilizer in 120 gallons of 60% solution of fertilizer,
which is  gallons of fertilizer. gallons of fertilizer.
So  or or  (which means the same in math class). (which means the same in math class).
That is your second equation.
You now have a system of 2 equations linear in two variables:

You can solve it by whatever method you chose.
For example, to solve by elimination, you may chose to multiply the first equation by 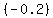 , getting , getting
 , ,
and add it to the second equation, getting
 --> -->  --> --> 
Now, from  and and  , you get , you get
 --> -->  --> --> 
NOTES FROM A CHEMIST
In real life, volume is often additive, so if you mix  gallons of a solution with gallons of a solution with  gallons of another solution, you get gallons of another solution, you get  gallons of mixture. (In math problems, volume is always additive).Chemists (like me) specify very clearly the concentration of a solution. gallons of mixture. (In math problems, volume is always additive).Chemists (like me) specify very clearly the concentration of a solution.
If we say 20% weight in weight (w/w), we mean that 100 g of solution contains 20 g of the item of interest.
If we say 20% weight in volume (w/v), we mean that 100 milliliters of solution contains 20 g of the item of interest.
If we say 20% volume in volume (v/v), we mean that 100 gallons of solution contains 20 gallons of a liquid of interest.
|
|
|