Question 1158751: 1. Mixture Problem
A grocer mixes peanuts that cost $1.49 per pound and walnuts that cost $2.69 per pound to make
100 pounds of a mixture that costs $2.21 per pound. How much of each kind of nut is put into the
mixture?
2. Mixture Problem
How much water must be evaporated from 80 pounds of a 12% solution of salt in order to obtain a
20% solution?
3. Investment Problem
A man invested Php 6,000, part of it at 3% and the rest at 4%. The total annual income from the
two investments is Php 208. How much is invested at each of these rates?
4. Pattern Problem
Determine the next number in the sequence 0, 10, 24, 56, 112, 190, ...
5. Pattern Problem
One cut of a stick of licorice produces two pieces. Two cuts produce three pieces. Three cuts
produce four pieces.
a. How many pieces are produced by five cuts and by six cuts?
b. Predict the nth-term formula for the number of pieces of licorice that are produced by n cuts
of a stick of licorice.
Answer by ikleyn(52786)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The RULE, the POLICY and the REQUIREMENT of this forum is
ONE and ONLY ONE problem/question per post.
Please DO NOT violate it.
Those who violate, work against their own interests.
We do not serve industrialists.
-------------------
I will solve only one problem for you.
2. Mixture Problem
How much water must be evaporated from 80 pounds of a 12% solution of salt in order to obtain a 20% solution?
Let W be the MASS of water to evaporate, in pounds.
The amount of salt in the original 80 pounds of the 12% solution was 0.12*80 = 9.6 pounds.
When W pounds of water were evaporated, the remaining mass became 80 pounds - W pounds.
We want the new salt concentration be 20%. It means
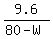 = 0.2. (1)
(Notice that when the water evaporates, the mass of salt REMAINS the SAME (!) )
To solve equation (1), multiply both sides by (80-W). You will get
9.6 = 0.2*(80-W).
Simplify and find W.
9.6 = 0.2*80 - 0.2W
0.2W = 0.2*80 - 9.6 = 6.4,
W = = 0.2. (1)
(Notice that when the water evaporates, the mass of salt REMAINS the SAME (!) )
To solve equation (1), multiply both sides by (80-W). You will get
9.6 = 0.2*(80-W).
Simplify and find W.
9.6 = 0.2*80 - 0.2W
0.2W = 0.2*80 - 9.6 = 6.4,
W = 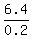 = 32 pounds.
ANSWER. 32 pounds of water should be evaporated.
CHECK. I will check equation (1) for the concentration = 32 pounds.
ANSWER. 32 pounds of water should be evaporated.
CHECK. I will check equation (1) for the concentration
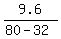 = = 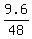 = 0.2, which is precisely correct (!) = 0.2, which is precisely correct (!)
Solved.
-------------------
It is a standard and typical mixture problem.
In this site, there is a bunch of lessons, covering various types of mixture problems. See introductory lessons
- Mixture problems
- More Mixture problems
- Solving typical word problems on mixtures for solutions
- Word problems on mixtures for antifreeze solutions
- Word problems on mixtures for alloys
- Typical word problems on mixtures from the archive
Read them and become an expert in solution the mixture word problems.
Also, you have this free of charge online textbook in ALGEBRA-I in this site
- ALGEBRA-I - YOUR ONLINE TEXTBOOK.
The referred lessons are the part of this online textbook under the topic "Mixture problems".
Save the link to this online textbook together with its description
Free of charge online textbook in ALGEBRA-I
https://www.algebra.com/algebra/homework/quadratic/lessons/ALGEBRA-I-YOUR-ONLINE-TEXTBOOK.lesson
to your archive and use it when it is needed.
|
|
|