Question 1155447: You need 270 mL of a 55% alcohol solution. On hand, you have a 50% alcohol mixture and a 80% alcohol mixture. How much of each mixture will you need to obtain the desired solution?
Found 2 solutions by josgarithmetic, ikleyn:
Answer by josgarithmetic(39630)   (Show Source): (Show Source):
You can put this solution on YOUR website! Just looking at the concentration values, see one part out of six parts from 50% to 80%; the wanted 55% is one part. You want most of the alcohol solution to come from the 50% and the rest from the 80%. Think 270*(5/6)=225 mL from the 50% and 45 mL from the 80%.
v, amount of the 80%
270-v, amount of the 50%
55, the target concentration percent wanted

after doing the algebra steps,
 -------volume of the 80% alcohol to use. -------volume of the 80% alcohol to use.
Answer by ikleyn(52914)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
Let x be the volume of the 80% mixture to mix (in mL),
Then the volume of the 50% mixture is (270-x) mL.
The total volume of the pure alcohol in ingredients is 0.8*x + 0.50*(270-x) mL,
and it should be equal to the volume of the pure alcohol in the mixture, which is 0.55*270.
So, your equation is
0.8*x + 0.50*(270-x) = 0.55*270 milliliters of the pure alcohol.
From the equation
x =  = 45.
ANSWER. 45 ml of the 80% mixture and the rest 270-45 = 225 ml of the 50% mixture.
CHECK. I will check for the concentration = 45.
ANSWER. 45 ml of the 80% mixture and the rest 270-45 = 225 ml of the 50% mixture.
CHECK. I will check for the concentration 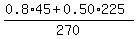 = 0.55 = 55%. ! Precisely correct ! = 0.55 = 55%. ! Precisely correct !
Solved.
------------------
There is a bunch of lessons in this site, covering various types of mixture problems. See introductory lessons
- Mixture problems
- More Mixture problems
- Solving typical word problems on mixtures for solutions
- Word problems on mixtures for antifreeze solutions
- Word problems on mixtures for alloys
- Typical word problems on mixtures from the archive
Read them and become an expert in solution the mixture word problems.
Also, you have this free of charge online textbook in ALGEBRA-I in this site
- ALGEBRA-I - YOUR ONLINE TEXTBOOK.
The referred lessons are the part of this online textbook under the topic "Mixture problems".
Save the link to this online textbook together with its description
Free of charge online textbook in ALGEBRA-I
https://www.algebra.com/algebra/homework/quadratic/lessons/ALGEBRA-I-YOUR-ONLINE-TEXTBOOK.lesson
to your archive and use it when it is needed.
|
|
|