.
mixing a given quantity of 30% silver alloy with a quantity of 90% silver yields 200 units of a 54% silver alloy.
How many units of each alloy were used?
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Let x = a mass of the 30% silver alloy used, and
y = a mass of the 90% silver alloy used.
Then the total mass equation is
x + y = 200,
and the "pure silver" mass equation is
0.3x + 0.9y = 0.54*200.
Simplify these equations and collect them into a system of equations
x + y = 200, (1)
0.3x + 0.9y = 108. (2)
To solve it, express x = 200 - y from (1) and substitute it into (2). You will get a single equation for y:
0.3*(200 - y) + 0.9y = 108,
60 - 0.3y + 0.9y = 108,
0.6y = 108 - 60 ---> 0.6y = 48 ---> y = 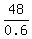 = 80.
Thus the mass of the 90% silver alloy used was 80 units.
The mass of the 30% silver alloy used was 200-80 = 120 units.
= 80.
Thus the mass of the 90% silver alloy used was 80 units.
The mass of the 30% silver alloy used was 200-80 = 120 units.