Question 1005270: Solution XRD is one half sulfur acid while QXJ is 3/4 sulfur acid. How many liters of solution QXJ should be added to a liter of solution XRD to obtain a solution that is 3/5 sulfur acid?
Found 3 solutions by josgarithmetic, MathTherapy, KMST:
Answer by josgarithmetic(39618)   (Show Source): (Show Source):
Answer by MathTherapy(10552)   (Show Source): (Show Source):
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! WHAT A MATH TEACHER THINKS:
The volume of a mix of two liquids is the sum of the volumes mixed together.

No need to specify concentration/amount units, because they are self-explanatory.
So the math teacher would add  Liters of solution QXJ to 1 Liter solution XRD and mix, expecting to get Liters of solution QXJ to 1 Liter solution XRD and mix, expecting to get 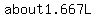 of solution with a 0.6 concentration. of solution with a 0.6 concentration.
WHAT A SCIENTIST THINKS:
Chemists know that it is important to specify your units.
When a chemist says a solution is 50% sulfuric acid,
they usually mean it contains 50 kg sulfuric acid per 100 kg solution.
If they want to be clearer, they say it is 50%(w/w).
Biologists tend to describe how the solutions were prepared.
When you mix together two substances, the mass of the mixture is the sum of the masses of the components.
The same is not true about volumes.
Scientists know that, and so do construction workers.
Any construction worker knows that mixing 1 cubic yard of rocks and 1 cubic yard of fine sand does not yield 2 cubic yards of mixture.
A chemist knows that mixing  Liter of a solution with Liter of a solution with  Liters of another solution yields a volume usually close to Liters of another solution yields a volume usually close to 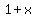 Liters, but not exactly Liters, but not exactly 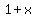 , and sometimes quite far from exactly that. , and sometimes quite far from exactly that.
A synthetic chemist and a biologist shrug and say it is probably close enough for their purposes.
An analytical chemist says that will make my analysis results very wrong.
IF A BIOLOGIST IS INVOLVED:
The way the concentrations of those solutions are described,
maybe those solutions are usually prepared by a biologist,
who mixes water and concentrated sulfuric acid to make solutions as follows:
Solution XRD "half sulfuric acid" = 1L water + 1L concentrated sulfuric acid;
Solution QXJ "3/4 sulfur acid" = 1L water + 3L concentrated sulfuric acid.
Maybe that biologist wanted to make more of
Solution UVW "3/5 sulfuric acid" = 2L water + 3L concentrated sulfuric acid.
The biologist most likely had a bottle of "concentrated sulfuric acid" that contained 98g of sulfuric acid per 100g of liquid.
The label may have stated "Assay....98.0%, meaning that its concentration was 98.0%(w/w).
Data in a handbook says that at   the density of that solution is the density of that solution is 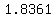 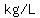 . .
Solution XRD, with  water and water and  concentrated acid, had a total mass of about concentrated acid, had a total mass of about
 , and , and
a total amount of pure acid of about  . .
(I say "about" because a biologist does not measure  volumes with anything near a precision of volumes with anything near a precision of 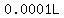 ). ).
The calculated acid concentration is  . .
The handbook says that the density of that solution is 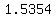 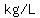 at at 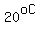  , ,
so the concentration is  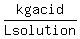 . .
Solution QXJ, with  water and water and  concentrated acid, had a total mass of about concentrated acid, had a total mass of about
 , and , and
about  of pure acid. of pure acid.
The calculated acid concentration is  . .
The handbook says that the density of that solution is 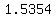 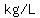 at at   , ,
so the concentration is  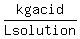 . .
Solution, with  water and water and
 concentrated acid, had a total mass of about concentrated acid, had a total mass of about
 , and , and
about  of pure acid. of pure acid.
The calculated acid concentration is  . .
The handbook says that the density of that solution is 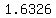 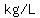 at at  , ,
so the concentration is  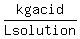 . .
Mixing  of solution XRD, of solution XRD,
containing 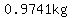 pure acid and pure acid and
  of Solution QXJ, of Solution QXJ,
containing  pure acid, the biologist gets pure acid, the biologist gets
a total of  pure acid in pure acid in
a total of  of solution. of solution.
The concentration is  in kg pure acid/kg solution, in kg pure acid/kg solution,
which still rounds to 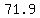 , close enough for the biologist. , close enough for the biologist.
The final volume of solution obtained is
 , also close enough for the biologist. , also close enough for the biologist.
IF A CHEMIST WAS DESCRIBING THOSE SOLUTIONS:
When a chemist says a solution is 50% sulfuric acid,
they usually mean it contains 50 kg sulfuric acid per 100 kg solution.
If they want to be clearer, they say it is 50%(w/w).
That is a pretty dense solution;
At 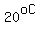 , ,  Liter has a mass of Liter has a mass of 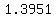 kg. kg.
When someone says a solution is 60% sulfuric acid,
they usually mean it contains 60 kg sulfuric acid per 100 kg solution.
If they want to be clearer, they say it is 60%(w/w).
That is a denser solution.
At 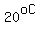 , ,  Liter has a mass of Liter has a mass of  kg. kg.
When someone says a solution is 75% sulfuric acid,
they usually mean it contains 75 kg sulfuric acid per 100 kg solution.
If they want to be clearer, they say it is 75%(w/w).
That is an even denser solution.
At 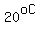 , ,  Liter has a mass of Liter has a mass of 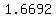 kg. kg.
If at 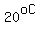 you measure you measure  Liter of 50% sulfuric acid solution, Liter of 50% sulfuric acid solution,
with a total mass of 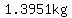 , containing , containing  sulfuric acid, and sulfuric acid, and
 Liter of 75% sulfuric solution with a mass of Liter of 75% sulfuric solution with a mass of  , containing , containing  sulfuric acid, you get sulfuric acid, you get
a total mass of  of solution containing of solution containing
 of sulfuric acid. of sulfuric acid.
The concentration of the resulting solution is
 . .
Looking in a handbook, we find that a solution with that final concentration will have a density of 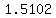  . .
So, the  of final solution will have a volume of of final solution will have a volume of
 . .
|
|
|