Question 169519: What is the pressure, in mm Hg, of 39.0 g of CO2 gas at 31°C, contained in a 350 mL vessel? (VII-1)
_____mm Hg
Answer by jojo14344(1513)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
It follows Ideal Gas Law -------->  , Working Eqn , Working Eqn
where ---> 
Solving for  : :

where, 
In 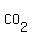 , there are "2" atoms of Oxygen and "1" atom of Carbon. , there are "2" atoms of Oxygen and "1" atom of Carbon.
Look on Periodic Table, we see  has Atomic Weight ---> has Atomic Weight ---> . Therefore, its Molecular Weight is expressed as . Therefore, its Molecular Weight is expressed as 
.
For Carbon, look on Periodic Table and the Atomic Weight is ----> . .
It's Molecular Weight is expressed as 
.
Therefore, 
Subst.: 

.
Going Back our Working Eqn,


 ----------> mmHg? ----------> mmHg?
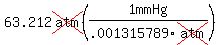
 , Answer , Answer
.
Thank you,
Jojo
|
|
|