Question 1201501: At time=0, there are 6,000 grams of a radioactive material present. The half-life of the element is 18 years. In how many years will there be 115 grams remaining? Round your answer to the nearest 0.01 years.
Any help would be appreciated, I have tried many problems like this one without success.
Found 3 solutions by math_tutor2020, josgarithmetic, MathTherapy:
Answer by math_tutor2020(3817)   (Show Source): (Show Source):
You can put this solution on YOUR website!
Answer: 102.69 years
Work Shown:
x = number of years
y = amount of substance leftover, in grams
One possible equation is
y = 6000*(0.5)^(x/18)
it is of the format
y = a*(0.5)^(x/H)
where 'a' is the starting amount and H is the half-life in years.
Plug in y = 115 and solve for x.
We'll need to use logs to isolate the exponent.
If the variable is in the trees, then we log it down.
y = 6000*(0.5)^(x/18)
115 = 6000*(0.5)^(x/18)
115/6000 = (0.5)^(x/18)
0.0191667 = (0.5)^(x/18)
Log(0.0191667) = Log( (0.5)^(x/18) )
Log(0.0191667) = (x/18)*Log( 0.5 ) ... use the logarithm power rule
x/18 = Log(0.0191667)/Log( 0.5 )
x = 18*Log(0.0191667)/Log( 0.5 )
x = 102.694576057311
x = 102.69
It takes roughly 102.69 years to have 115 grams of substance remaining.
Another problem involving half-life.
https://www.algebra.com/algebra/homework/word/misc/Miscellaneous_Word_Problems.faq.question.1201494.html
Answer by josgarithmetic(39630)   (Show Source): (Show Source):
Answer by MathTherapy(10557)   (Show Source): (Show Source):
You can put this solution on YOUR website!
At time=0, there are 6,000 grams of a radioactive material present. The half-life of the element is 18 years. In how many years will there be 115 grams remaining? Round your answer to the nearest 0.01 years.
Any help would be appreciated, I have tried many problems like this one without success.
If ½-life is “a” time-periods, then k, or DECAY CONSTANT =  CONTINUOUS GROWTH/DECAY formula:
CONTINUOUS GROWTH/DECAY formula:  , with: , with:
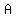 being remaining amount after time t (115, in this case) being remaining amount after time t (115, in this case)
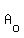 being Original/Initial amount (6,000, in this case) being Original/Initial amount (6,000, in this case)
 being the constant (k > 0 signifies RATE OF GROWTH ; k < 0 signifies RATE OF DECAY ; k = - .0385, in this case) being the constant (k > 0 signifies RATE OF GROWTH ; k < 0 signifies RATE OF DECAY ; k = - .0385, in this case)
 being time, in stated periods (Unknown, in this case) being time, in stated periods (Unknown, in this case)

 ----- Substituting 115 for A, 6,000 for ----- Substituting 115 for A, 6,000 for 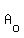 , and - .0385 for k , and - .0385 for k

 ------ Converting to LOGARITHMIC (Natural) form
Time it takes for 115 grams to remain, or ------ Converting to LOGARITHMIC (Natural) form
Time it takes for 115 grams to remain, or  The correct answer should actually be in WHOLE-NUMBER years (103 to be specific)!
The correct answer should actually be in WHOLE-NUMBER years (103 to be specific)!
|
|
|