Question 1085178: A) Equal masses of water at 20 Celsius and iron at 40 Celsius are put together in a Styrofoam cup. The water has a specific heat of 1 cal/g C and the iron has a specific heat of 0.113 cal/g C. What do you think the final temp will be of both the water and the iron in the Styrofoam cup? Show your work.
B) What would happen if you combined equal masses of liquid water at 10 C with ice at 0 C? What do you think the final temp might be?
Found 3 solutions by rothauserc, ikleyn, KMST:
Answer by rothauserc(4718)   (Show Source): (Show Source):
You can put this solution on YOUR website! A) Let x be the final temperature of the mixture
:
The iron is warmer, so its delta temperature is 40 - x
:
The water's delta temperature is x - 20
:
the energy amount going out of the iron is equal to the energy amount going into the cool water. This means,
:
q(lost) = q(gain)
:
we are given that the mass(m) of the iron is the same as the water
:
we know
:
q = m * delta temperature * specific heat
:
m * (40 - x) * 0.113 = m * (x - 20) * 1
:
now we solve for x
:
4.52 - 0.113x = x - 20
:
-1.113x = -24.52
:
x = 8.1222
:
*****************************
final temperature is 8.1222 C
*****************************
:
B) Our balance equation, q(lost) = q(gain) is
:
m * (10 - x) * 1 = m * (x - 0) * 1
:
10 - x = x
:
2x = 10
:
x = 5
:
*****************************************
we expect the final temperature to be 5 C
*****************************************
:
Answer by ikleyn(52775)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
A) Equal masses of water at 20 Celsius and iron at 40 Celsius are put together in a Styrofoam cup. The water has a specific heat of 1 cal/g C and the iron has a specific heat of 0.113 cal/g C. What do you think the final temp will be of both the water and the iron in the Styrofoam cup? Show your work.
B) What would happen if you combined equal masses of liquid water at 10 C with ice at 0 C? What do you think the final temp might be?
~~~~~~~~~~~~~~~~~~
A) The correct answer is THIS:
Final temperature of the system is 22.03 °C.
B) Solution B) by "rothauserc" is totally and conceptually wrong, since the latent heat of the ice melting is not accounted.
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! A) If  is the mass, in grams, of each component (water and iron, is the mass, in grams, of each component (water and iron,
and  is the final temperature of both the water and the iron, in degrees Celsius, is the final temperature of both the water and the iron, in degrees Celsius,
the heat gained by the water, in calories, is
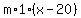 , ,
and the heat lost by the iron is
 . .
working in a Styrofoam cup the heat gained from or lost to the environment is negligible, so
 <--> <-->  . .
Solving:






B) It takes 79.72 calories to melt 1 gram of ice.
That is called enthalpy of fusion, or heat of fusion, or latent heat of fusion.
It is a lot of heat, so ice can take a lot of heat away from anything it touches.
Can 1 gram of ice take enough heat from 1 gram of water to cool it from 10 degrees to 0 degrees?
Let's see.
Cooling 1 gram of water from 10 degrees to 0 degrees (Celsius) takes only
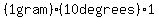  . .
So, if you mix 1 gram of water at 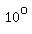  and 1 gram of ice at and 1 gram of ice at   , ,
you get water at   with a slightly smaller amount of ice floating in it, all of it at with a slightly smaller amount of ice floating in it, all of it at   . .
|
|
|