.
Liquid A has 10 grams of element X per liter. Liquid B has 15 milligrams of element Y per cm3
(there are 1000 milligrams in a gram and 1000 cm3 in a liter).
It is desired to make 150 grams of molecule Z. To make 18 grams of Z requires 2 grams of X and 16 grams of Y.
How many liters of each liquid should be used?
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Liquid A has 10 grams of element X per liter.
Liquid B has 15 milligrams of element Y per cm3, or, which is the same,
15 gram of element Y per liter.
To make 18 grams of Z requires 2 grams of X and 16 grams of Y, or, which is the same,
0.2 liters of A and  liters of B. (*)
liters of B. (*)
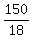 =
= 
 .
Therefore, the amounts (*) must be multiplied by
.
Therefore, the amounts (*) must be multiplied by 
 =
= 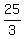 each.
So, you need
each.
So, you need  liters of A and
liters of A and  liters of B.
Again:
liters of B.
Again:  =
= 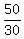 =
=  liters of A, and
liters of A, and
 =
=  liters of B.
liters of B.
Solved.