Question 540432: PH=-log[h]
if Ph=5.7
how we can calculate the log[h]
I need the answer with explanation.thank u
Answer by KMST(5328)   (Show Source): (Show Source):
You can put this solution on YOUR website! pH=-log[H^+] is calculated based on the molar concentration of the positively charged hydrogen ion. (The + or - charges for ions are written as superscripts, as if they were exponents. The p is always lowercase, never a capital letter, while H and O are always capital letters).
If pH=-log([H])=5.7 --> log([H])=-5.7 and [H]=
[H+]=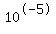 <--> pH=5, [H+]= <--> pH=5, [H+]=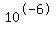 <--> pH=6, If the concentration is somewhere in between, so is the pH, but the higher [H+] the lower the pH. <--> pH=6, If the concentration is somewhere in between, so is the pH, but the higher [H+] the lower the pH.
pH=7 is neutral, pH<7 is acidic (excess H+ ions), and pH>7 is alkaline (also called basic).
BECAUSE I AM A CHEMIST AFTER ALL:
Water is dissociated to a very, very limited extent into H+ and OH- ions. There is an equilibrium between the whole molecules on one side and the ions on the other.
In pure water there are 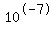 moles per liter of each ion, and the product of those concentrations is moles per liter of each ion, and the product of those concentrations is 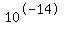 . The pH of that pure water would be . The pH of that pure water would be
 . That is called a neutral pH. . That is called a neutral pH.
If you add to that water a compound that will provide more H+ or OH- ions, the concentrations will change, and some of the H+ and OH-ions will join to form more water molecules. The result will be new values for the molar concentrations of H+ and OH-, but those concentrations, multiplied will still be 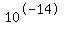 . That number is a constant that is called the ionic product of water. . That number is a constant that is called the ionic product of water.
If you add an acid the concentration of H+ will increase. The pH and the concentration of OH- will decrease.
You may end up with [H+]=0.01=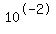 (pH=2) and [OH-]= (pH=2) and [OH-]= . .
If you add a base, like sodium hydroxide (NaOH), the concentration of OH- and the pH will increase, while the concentration of H+ will decrease.
You may end up with [OH-]=0.001=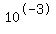 , [H+]= , [H+]=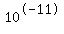 and pH=11 and pH=11
|
|
|