Question 1195767: An archeologist uses radiocarbon dating to determine the age of a Viking ship. Suppose a
sample that originally contained 100 mg of Carbon-14 now contains 85 mg. If the half-life of
Carbon-14 is 5730 years, what is the age of the ship to the nearest 100 years?
Found 2 solutions by Theo, ikleyn:
Answer by Theo(13342)   (Show Source): (Show Source):
You can put this solution on YOUR website! one formula you can use is f = p * (1 + r) ^ n
f is the future value.
p is the present value.
(1+r) is the growth factor.
n is the number of years.
use the number of years for the half life to find (1+r)
the formula will become:
.5 = (1+r) ^ 5730
solve for (1+r) to get:
(1+r) = .5 ^ (1/5730) = .9998790392.
when 100 becomes 85, the formula becomes:
85 = 100 * (1+r) ^ n
divide both sides of this equation by 100 to get:
85/100 = (1+r) ^ n
take the log of both sides of this equation to get:
log(85/100) = log((1+r) ^ n)
this becomes:
log(85/100) = n * log(1+r) by the log rule that says log(x ^ n) = n * log(x).
the equation becomes:
log(85/100) = n * log(1+r)
divide both sides of this equation by log(1+r) and solve for n to get:
n = log(85/100) / log(1+r) = log(85/100) / log(.9998790392) = 1343.485903.
to confirm, replace n in the original equation with .9998790392 and n with 1343.485903to get:
f = 100 * (.9998790392) ^ 1343.485903 = 85.
this confirms the n is good.
round the value of n to the nearest 100 years to get:
1343.485903 = 1300
the equation can be graphed as shown below:

let me know if you have any questions.
theo
Answer by ikleyn(52781)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
An archeologist uses radiocarbon dating to determine the age of a Viking ship. Suppose a
sample that originally contained 100 mg of Carbon-14 now contains 85 mg. If the half-life of
Carbon-14 is 5730 years, what is the age of the ship to the nearest 100 years?
~~~~~~~~~~~~~~~~~~
I do not know who is a composer of this problem - you or your professor or the third party,
but from the post, I definitely know that this person DOES NOT know the basics of the underlying
decay process and the decay dating method.
In the natural organic samples, the initial ratio of the masses of radioactive C-14 to stable C-12 is about 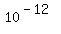 . .
It is even less than tiny percent.
Nevertheless, the existing measuring tools allow accurately measure this ratio.
With time, the amount of C-14 decreases exponentially, and the ratio of masses C-14 to C-12 decreases accordingly.
In the dating method, ONLY THIS RATIO is the subject of measuring and consideration.
In order for the sample could contain 100 mg (one hundred milligrams) of C-14, the sample mass should be
 = =  milligrams = milligrams =  milligrams = milligrams = 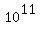 grams = grams = 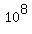 kilograms = kilograms = 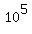 metric tons metric tons
or 100,000 metric tons.
Therefore, NOBODY and NEVER on the Earth had an organic 100 mg sample of C-14.
So, you better throw this " problem " to the closest garbage bin and do not disgrace yourself.
---------------
About Carbon-14 decay dating method, learn from these authoritative sources
https://www2.lbl.gov/abc/wallchart/chapters/13/4.html
https://en.wikipedia.org/wiki/Radiocarbon_dating
To see many problems on Carbon-14 decay dating method, look into the lessons
- Using logarithms to solve real world problems, and
- Carbon dating problems
Also, you have this free of charge online textbook in ALGEBRA-I in this site
- ALGEBRA-I - YOUR ONLINE TEXTBOOK.
The referred lessons are the part of this online textbook under the topic "Logarithms".
Save the link to this online textbook together with its description
Free of charge online textbook in ALGEBRA-I
https://www.algebra.com/algebra/homework/quadratic/lessons/ALGEBRA-I-YOUR-ONLINE-TEXTBOOK.lesson
to your archive and use it when it is needed.
|
|
|