.
Let x be the volume of the premium 85% antifreeze (in gallons) and
let y be the volume of water.
x + y = 170 gallons (1) (total volume equation)
0.85x = 0.15*170 gallons (2) (pure antifreeze volume equation)
From equation (2), x =  = 30.
Then from (1), y = 170-30 = 140 gallons.
ANSWER. 140 gallons of water.
= 30.
Then from (1), y = 170-30 = 140 gallons.
ANSWER. 140 gallons of water.
The same solution can be presented in wording form.
Since they simply DILUTE the existing mixture, the content of the pure antifreeze remains the same: it is  = 25.5 gallons.
Hence, the 85% premium antifreeze should have the volume of
= 25.5 gallons.
Hence, the 85% premium antifreeze should have the volume of 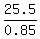 = 30 gallons to be 85% mixture.
The complement to 170 gallons, i.e. 170 - 30 = 140 gallons, is the water volume, which should be added.
= 30 gallons to be 85% mixture.
The complement to 170 gallons, i.e. 170 - 30 = 140 gallons, is the water volume, which should be added.
You get the same answer.