Question 1190602: The hydrogen (H) in a container of C6H8NCL weighs 80 grams. How much does the nitrogen (N) in the container weigh? (C,12; H,1; N,14; CL,35)
Answer by ikleyn(52781)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The hydrogen (H) in a container of C6H8NCL weighs 80 grams. How much does the nitrogen (N)
in the container weigh? (C,12; H,1; N,14; CL,35)
~~~~~~~~~~~~~
Based on chemical formula, there are 14 grams of nitrogen (N) for every 8 gram of hydrogen in this chemical.
THEREFORE, for 80 grams of hydrogen, there are 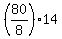 = 10*14 = 140 grams of nitrogen (N). ANSWER = 10*14 = 140 grams of nitrogen (N). ANSWER
Solved and explained.
---------------
Last time I studied Chemistry about ...ty years ago, but still remember the basics.
By the way, there is a lesson in this site on proportions in Chemistry
- Using proportions to solve Chemistry problems
in this site.
It is written specially for you.
Read it and become an expert in this area.
Consider this lesson as your textbook, handbook, tutorials and (free of charge) home teacher.
|
|
|