.
In terms of the half-life, the general formula for radioactive decay of the Carbon-14 is
C(t) =  .
.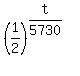 where C(t) is the current mass of the carbon-14; C(0) is the initial mass,
Since 335% of the Carbon-14 remained, you have this equation
0.35*C(0) =
where C(t) is the current mass of the carbon-14; C(0) is the initial mass,
Since 335% of the Carbon-14 remained, you have this equation
0.35*C(0) =  .
.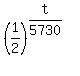 , which reduces to 0.35 =
, which reduces to 0.35 = 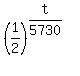 ,
To solve it, take logarithm base 10 from both sides. You get an equation
,
To solve it, take logarithm base 10 from both sides. You get an equation
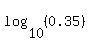 =
= 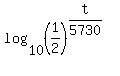 , or
, or 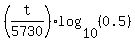 =
= 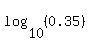 .
Therefore,
t =
.
Therefore,
t = 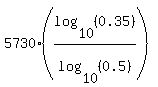 = 8768 years.
ANSWER. The piece of wood is about 8768 years old.
= 8768 years.
ANSWER. The piece of wood is about 8768 years old.
Solved.
----------------
The post-solution note:
Since the half-life parameter is given, it is NATURALLY to have the ENTIRE SOLUTION in half-life terms.
The transition to other form (to "ekt-form"), as @josgaritmetic starts his solution, is not necessary.
It only leads to unnecessary excessive job and creates the room for errors.