Question 1187771: The half life of a sodium is 15 hours. How long does it take for 95% of a sample of this
isotope to decay?
Answer by ikleyn(52792)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
The half life of a sodium is 15 hours. How long does it take for 95% of a sample of this
isotope to decay?
~~~~~~~~~~~~~~~~~~
If 95% of the material decayed, it means that 5% of the material remained.
So we write the decay equation in terms of half-life
0.05 = 0.5^(t/15),
where "t" is the sough time, in hours.
Next we take the logarithm base 10 from both sides
log(0.05) = 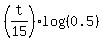 ,
and we get the ANSWER
t = ,
and we get the ANSWER
t = 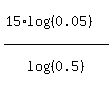 = use your calculator = 64.82892142 hours = 64.829 hours (rounded). = use your calculator = 64.82892142 hours = 64.829 hours (rounded).
Solved.
------------------
On radioactive decay, see the lesson
- Radioactive decay problems
in this site.
You will find many similar (and different) solved problems there.
Use this lesson as your handbook, textbook, guide, tutorials, and (free of charge) home teacher.
Learn the subject from there once and for all.
Also, you have this free of charge online textbook in ALGEBRA-I in this site
- ALGEBRA-I - YOUR ONLINE TEXTBOOK.
The referred lesson is the part of this online textbook under the topic "Logarithms".
Save the link to this online textbook together with its description
Free of charge online textbook in ALGEBRA-I
https://www.algebra.com/algebra/homework/quadratic/lessons/ALGEBRA-I-YOUR-ONLINE-TEXTBOOK.lesson
to your archive and use it when it is needed.
|
|
|